Maintaining the potency and efficacy of vaccines is paramount to public health. A critical component of this is the meticulous and precise storage of these life-saving biological products. Improper storage, particularly temperature excursions outside the recommended range, can lead to irreversible damage, rendering vaccines ineffective and jeopardizing immunization efforts. This comprehensive guide delves deep into the intricacies of refrigerator vaccine storage, providing an exhaustive overview of best practices, regulatory requirements, and the selection of appropriate refrigeration solutions. We will explore every facet, from understanding the fundamental principles of the vaccine cold chain to implementing robust monitoring systems that guarantee the integrity of your valuable vaccine inventory.
Understanding the Critical Importance of Correct Vaccine Storage Temperatures
Vaccines are delicate biological preparations that are highly sensitive to temperature fluctuations. Exposure to temperatures outside the manufacturer’s specified range can compromise their immunogenicity, meaning they will no longer be able to elicit the intended immune response in the recipient. This can have severe consequences, leading to inadequate protection against preventable diseases and undermining public trust in vaccination programs. Therefore, adhering strictly to recommended storage temperatures within specialized vaccine refrigerators is not merely a guideline; it is an absolute necessity for ensuring patient safety and the effectiveness of immunization campaigns.
The consequences of improper storage extend beyond just a loss of efficacy. In some cases, exposure to excessive heat or freezing temperatures can alter the vaccine composition in ways that may even pose a risk to the patient. Furthermore, the financial implications of vaccine wastage due to improper storage can be substantial for healthcare providers and public health agencies. Investing in and diligently maintaining appropriate vaccine storage refrigerators is a crucial investment in both patient well-being and operational efficiency.
Navigating Regulatory Guidelines for Vaccine Refrigerator Storage
Numerous regulatory bodies and professional organizations provide stringent guidelines for the storage and handling of vaccines. These guidelines are designed to ensure the integrity of the vaccine cold chain from the point of manufacture to the point of administration. Key organizations such as the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) publish detailed recommendations on temperature ranges, storage unit specifications, monitoring protocols, and inventory management. Compliance with these guidelines is not only a legal obligation in many jurisdictions but also a fundamental ethical responsibility for healthcare providers.
Understanding and adhering to these regulations is crucial for accreditation, audits, and maintaining the confidence of patients and the wider community. This includes meticulous record-keeping of temperature logs, regular maintenance of vaccine storage refrigerators, and the implementation of robust protocols for handling temperature excursions. Staying updated on the latest guidelines and recommendations from relevant authorities is an ongoing process that is essential for all stakeholders involved in vaccine administration.
Key Features to Look for in a Dedicated Vaccine Storage Refrigerator
Not all refrigerators are created equal when it comes to storing vaccines. Standard domestic or even commercial refrigerators are often unsuitable due to their temperature fluctuations and lack of features designed specifically for the sensitive nature of vaccines. Investing in a purpose-built medical-grade or pharmaceutical-grade refrigerator is a critical step in ensuring optimal storage conditions. These specialized units are engineered with features that address the unique requirements of vaccine storage:
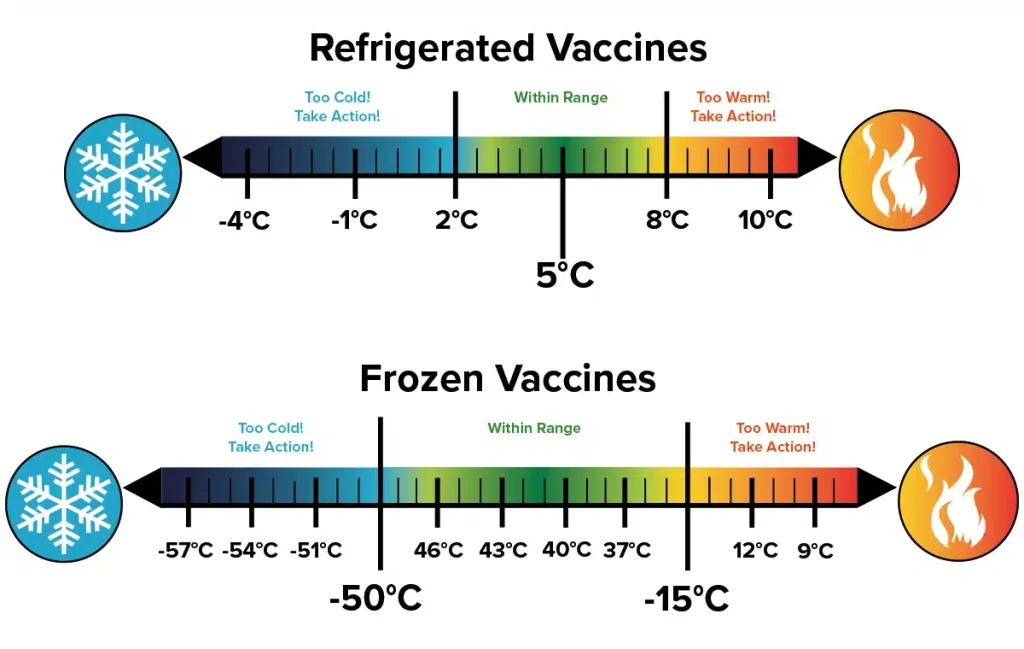
- Precise Temperature Control: Dedicated vaccine refrigerators offer highly accurate and stable temperature control within the recommended range (typically between 2°C and 8°C or 36°F and 46°F). They often feature digital temperature displays and sophisticated control systems to minimize fluctuations.
- Forced-Air Circulation: Uniform temperature distribution throughout the storage compartment is essential to prevent hot or cold spots that could compromise vaccine efficacy. Forced-air circulation systems ensure consistent temperatures on all shelves and in all areas of the refrigerator.
- Alarm Systems: Integrated alarm systems provide immediate notification of temperature excursions outside the acceptable range, allowing for prompt corrective action to be taken. These alarms can be audible and visual, and some advanced systems offer remote monitoring and alerts.
- Data Logging Capabilities: Many pharmaceutical-grade refrigerators include built-in data loggers that continuously record temperature data. This information is crucial for demonstrating compliance with regulatory requirements and for identifying any potential issues with temperature control over time.
- Secure Locking Mechanisms: To prevent unauthorized access and ensure the security of valuable vaccine inventory, dedicated units often feature secure locking mechanisms.
- Optimized Storage Configurations: The internal design of vaccine storage refrigerators is often optimized for efficient organization and airflow, with adjustable shelves and designated compartments.

When selecting a refrigerator for vaccine storage, it is imperative to prioritize these features to guarantee the integrity and efficacy of your vaccine supply.
Best Practices for Organizing and Managing Vaccines Within the Refrigerator
Even with a high-quality vaccine refrigerator, proper organization and management are crucial for maintaining optimal storage conditions and preventing errors. Implementing the following best practices can significantly enhance the safety and efficacy of your vaccine inventory:
- Designated Storage: Vaccines should be stored separately from other medical supplies or laboratory specimens to minimize the risk of contamination and ensure easy access.
- Proper Spacing: Avoid overcrowding the refrigerator, as this can impede airflow and lead to inconsistent temperatures. Ensure adequate spacing between vaccine packages to allow for proper air circulation.
- Strategic Placement: Follow manufacturer guidelines for optimal placement of different vaccine types within the refrigerator. Avoid storing vaccines in doors, where temperature fluctuations are more likely.
- First-In, First-Out (FIFO): Implement a FIFO system to ensure that vaccines are used before their expiration dates. Regularly check expiration dates and remove expired vaccines promptly.
- Regular Inventory Checks: Conduct regular inventory checks to reconcile the physical stock with inventory records and identify any discrepancies or potential issues.
- Emergency Preparedness: Develop and maintain a comprehensive emergency plan in case of power outages or refrigerator malfunctions. This plan should include procedures for temporarily storing vaccines in alternative temperature-controlled environments.
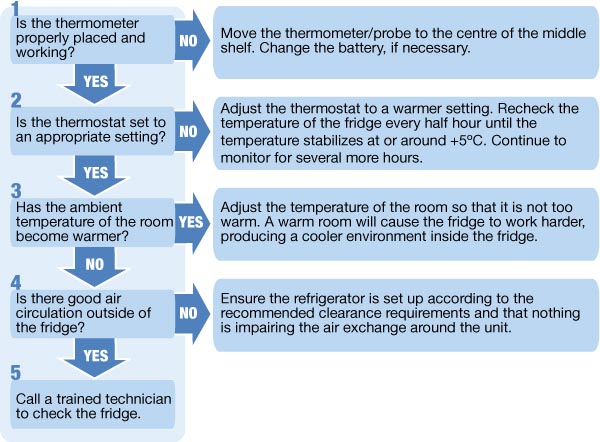
Adhering to these organizational principles is essential for maintaining an efficient and safe vaccine storage system.
The Importance of Continuous Temperature Monitoring and Documentation
Temperature monitoring is the cornerstone of effective vaccine storage. Relying solely on the refrigerator’s internal thermostat is insufficient, as it only reflects the temperature at the sensor location, not necessarily throughout the entire storage compartment. Implementing a robust temperature monitoring system is crucial for detecting and addressing any temperature excursions promptly.
Utilizing calibrated temperature monitoring devices (TMDs), such as digital data loggers or continuous temperature monitoring systems, is highly recommended. These devices provide accurate and continuous temperature readings, allowing for the identification of even brief temperature fluctuations that could compromise vaccine integrity. Regular review of temperature logs is essential, and any deviations from the recommended range must be investigated and documented thoroughly, along with any corrective actions taken.
Detailed documentation of temperature logs, maintenance records, inventory checks, and any temperature excursions is not only a regulatory requirement but also a vital tool for ensuring accountability and identifying trends that may indicate potential problems with the vaccine storage refrigerator or storage protocols.
Responding Effectively to Vaccine Refrigerator Temperature Excursions
Despite the best efforts, temperature excursions outside the recommended range can occasionally occur. Having a well-defined protocol for responding to these events is critical to minimizing potential damage and ensuring patient safety. This protocol should include the following steps:
- Immediate Identification: Promptly recognize and acknowledge the temperature excursion based on alarm notifications or review of temperature logs.
- Isolation of Affected Vaccines: Immediately isolate the affected vaccines and clearly label them as “Do Not Use” until their viability can be determined.
- Investigation of the Cause: Thoroughly investigate the cause of the temperature excursion to prevent future occurrences. This may involve checking the refrigerator’s settings, power supply, door seals, and ambient temperature.
- Consultation with Experts: Contact the vaccine manufacturer and/or relevant public health authorities for guidance on the viability of the affected vaccines. Do not discard or administer potentially compromised vaccines without expert consultation.
- Detailed Documentation: Document the temperature excursion, the investigation findings, the consultation process, and the final disposition of the affected vaccines.
- Corrective Actions: Implement any necessary corrective actions to address the underlying cause of the temperature excursion, such as repairing or replacing the refrigerator, adjusting storage protocols, or providing additional staff training.
A proactive and well-rehearsed response plan can mitigate the risks associated with temperature excursions and protect the integrity of your vaccine supply.
The Long-Term Value of Investing in High-Quality Vaccine Refrigeration Solutions
While the initial investment in a dedicated medical-grade or pharmaceutical-grade refrigerator may seem significant, the long-term benefits far outweigh the costs. Ensuring the efficacy and safety of your vaccine supply protects patient health, reduces vaccine wastage, minimizes the risk of regulatory non-compliance, and enhances the reputation of your healthcare facility. A reliable vaccine storage refrigerator is an indispensable asset in any setting where vaccines are handled and administered.
Furthermore, modern vaccine refrigerators are often designed with energy efficiency in mind, leading to lower operating costs over the lifespan of the unit. The advanced features, such as precise temperature control, alarm systems, and data logging capabilities, provide peace of mind and streamline the management of your valuable vaccine inventory. Choosing a reputable manufacturer with a proven track record of reliability and customer support is also crucial for a long-term, successful investment in refrigerator vaccine storage.
Conclusion: Upholding the Integrity of the Vaccine Cold Chain Through Diligent Refrigerator Storage Practices
The proper storage of vaccines in dedicated refrigerators is a fundamental pillar of public health. By adhering to regulatory guidelines, investing in appropriate refrigeration equipment, implementing best practices for organization and management, and maintaining meticulous temperature monitoring and documentation, healthcare providers can ensure the potency, efficacy, and safety of these life-saving biological products. The information presented in this comprehensive guide underscores the critical importance of every step in the vaccine cold chain, with refrigerator vaccine storage playing a pivotal role in safeguarding the health and well-being of communities worldwide. Embracing a culture of vigilance and continuous improvement in vaccine storage practices is not just a responsibility; it is a commitment to protecting public health and ensuring the success of immunization programs for generations to come. The selection and diligent management of your vaccine storage refrigerator are therefore not merely operational tasks, but crucial acts of ensuring a healthier future for all.