In the intricate world of healthcare, few elements are as critical yet often understated as the precise temperature at which vaccines are stored. Maintaining the integrity of these life-saving biological preparations is paramount, directly impacting their efficacy and, ultimately, the health and well-being of individuals and communities. This comprehensive guide delves deep into the essential aspects of vaccine refrigerator temperature, exploring optimal ranges, the science behind temperature sensitivity, stringent monitoring protocols, and the potentially dire consequences of temperature excursions. Understanding and adhering to best practices in vaccine cold chain management is not merely a recommendation; it is a fundamental responsibility for all healthcare providers and facilities involved in vaccine storage and administration.
The Critical Importance of Precise Vaccine Storage Temperature
Vaccines are complex biological products composed of delicate antigens, adjuvants, and stabilizers. These components are highly sensitive to temperature fluctuations. Exposure to temperatures outside the recommended range, whether too hot or too cold, can lead to irreversible damage, compromising the vaccine’s potency and rendering it ineffective. Administering a compromised vaccine not only fails to provide the intended protection against disease but also erodes public trust in vaccination programs. Therefore, maintaining the correct vaccine refrigerator temperature is not just about compliance; it is about safeguarding public health and ensuring the investment in vaccine development and distribution yields its intended benefits.
- Maintaining Efficacy: Correct temperature preserves the structural integrity of vaccine components, ensuring they elicit the desired immune response.
- Preventing Degradation: Exposure to inappropriate temperatures can lead to chemical and physical degradation of the vaccine, reducing its effectiveness.
- Ensuring Patient Safety: Administering a vaccine that has lost potency due to improper storage leaves individuals vulnerable to preventable diseases.
- Regulatory Compliance: Strict guidelines and regulations mandate precise temperature control throughout the vaccine supply chain.
Understanding the Ideal Vaccine Refrigerator Temperature Range
The generally recommended temperature range for the storage of most refrigerated vaccines is between 2°C and 8°C (36°F and 46°F). This narrow window is crucial for maintaining their stability and efficacy. It is imperative to understand that this is not a target temperature but a strict range that must be consistently maintained. Some vaccines may have specific storage requirements that fall within or slightly outside this general range, so always refer to the manufacturer’s guidelines and the specific recommendations from your local health authorities (e.g., the Centers for Disease Control and Prevention (CDC) in the United States or the World Health Organization (WHO) globally). Deviations from this recommended temperature range, even for short periods, can have detrimental effects.
- The Danger of Freezing: Freezing temperatures can be particularly damaging to some vaccines, altering their composition and rendering them unusable.
- The Risk of Overheating: Temperatures above 8°C can accelerate the degradation of vaccine components, leading to a loss of potency.
- Manufacturer Specifications: Always consult the product insert for specific storage instructions, as some vaccines may have unique temperature requirements.
Implementing Robust Vaccine Temperature Monitoring Protocols
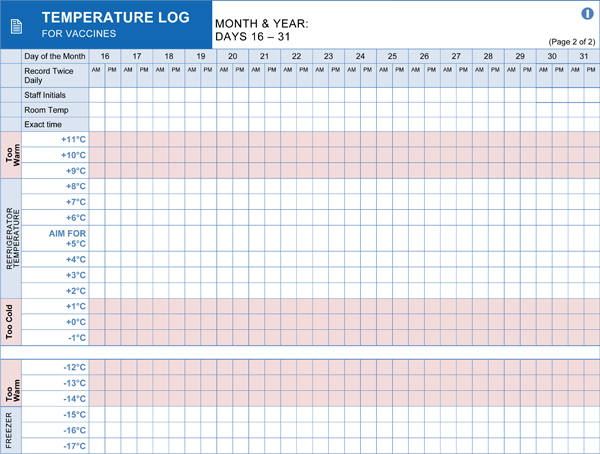
Passive storage alone is insufficient to guarantee that vaccines remain within the required temperature range. Implementing a comprehensive temperature monitoring system is essential. This involves utilizing calibrated temperature monitoring devices and establishing consistent recording practices. Continuous monitoring provides a detailed history of storage conditions, allowing for timely intervention if temperature excursions occur. Regular review of temperature logs is crucial for identifying trends and ensuring the refrigerator is functioning correctly. Investing in reliable and accurate temperature monitoring equipment is a cornerstone of effective vaccine management.
- Using Calibrated Devices: Employ digital data loggers or continuous temperature monitoring devices with accurate probes. Ensure regular calibration of these devices.
- Multiple Readings: Take temperature readings at least twice daily, preferably at the beginning and end of the workday. Some guidelines recommend more frequent monitoring.
- Documenting Readings: Maintain detailed and accurate temperature logs, including the date, time, and the initials of the person taking the reading.
- Buffer Solutions: Place temperature probes in a buffered solution (e.g., glycol or glass beads) to better reflect the actual temperature of the stored vaccines rather than rapid air temperature fluctuations.
Selecting and Maintaining Appropriate Vaccine Refrigeration Equipment
Not all refrigerators are suitable for storing vaccines. Standard household refrigerators are generally not recommended due to inconsistent temperature control and the risk of freezing in certain areas. Purpose-built pharmaceutical-grade refrigerators or medical-grade refrigerators are designed to maintain a stable and uniform temperature throughout the unit. These refrigerators often feature forced-air circulation, digital temperature displays, and alarm systems to alert staff to temperature excursions. Regular maintenance of the vaccine refrigerator is also crucial to ensure its proper functioning and longevity. This includes regular cleaning, defrosting (if it’s not a frost-free model), and professional servicing as recommended by the manufacturer.
- Pharmaceutical-Grade Refrigerators: These offer superior temperature control and stability.
- Dedicated Units: Ideally, vaccines should be stored in a dedicated refrigerator to minimize temperature fluctuations caused by frequent opening and closing.
- Proper Loading: Avoid overcrowding the refrigerator, which can impede air circulation and lead to uneven temperatures. Leave space between vaccine packages.
- Regular Maintenance: Follow the manufacturer’s guidelines for cleaning and maintenance. Schedule regular professional servicing.
Responding Effectively to Vaccine Temperature Excursions
Despite diligent monitoring and maintenance, temperature excursions can occasionally occur. It is crucial to have a well-defined protocol in place for responding to these events. This protocol should outline the steps to take immediately upon discovering an excursion, including isolating the affected vaccines, documenting the details of the excursion (date, time, duration, and the extent of the temperature deviation), and contacting the relevant authorities (e.g., the vaccine manufacturer and the local health department) for guidance on the viability of the affected vaccines. Never administer vaccines that have been exposed to temperatures outside the recommended range without explicit guidance from these authorities. Proper documentation of temperature excursions is vital for quality assurance and regulatory compliance.
- Immediate Action: Isolate the affected vaccines and clearly label them “Do Not Use.”
- Detailed Documentation: Record all relevant information about the excursion.
- Consultation: Contact the vaccine manufacturer and local health authorities for guidance.
- Quarantine Protocol: Establish a designated quarantine area for vaccines that have experienced temperature excursions.
The Broader Impact of Maintaining Optimal Vaccine Refrigerator Temperature
The seemingly simple act of maintaining the correct vaccine refrigerator temperature has profound implications that extend far beyond the individual vial. It underpins the success of immunization programs, contributes to disease eradication efforts, and fosters public confidence in the healthcare system. When the vaccine cold chain is robust and meticulously managed, it ensures that every administered dose is potent and effective, providing the intended protection against preventable diseases. Conversely, failures in temperature control can lead to vaccine wastage, increased healthcare costs, and, most importantly, a diminished ability to protect vulnerable populations. Investing in and prioritizing proper vaccine storage practices is an investment in public health and a testament to a commitment to quality care.
- Public Health Protection: Ensures the effectiveness of immunization programs.
- Economic Efficiency: Reduces vaccine wastage and associated costs.
- Building Trust: Reinforces public confidence in vaccination efforts.
- Global Health Security: Contributes to global disease prevention and control.
This comprehensive guide highlights the critical importance of maintaining the correct vaccine refrigerator temperature. Adherence to these best practices is essential for ensuring vaccine potency, patient safety, and the overall success of immunization programs. Always consult official guidelines and manufacturer recommendations for specific vaccine storage requirements.